Bio & Pharma
LG Chem receives FDA nod for CUE-102 cancer treatment phase 1 trials
LG Chem’s life science affiliate has 12 drug candidates in the pipeline, of which four are anticancer therapies
By May 13, 2022 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



LG Chem Life Sciences, the biopharmaceutical unit of LG Chem Ltd., has received the US Food and Drug Administration’s approval for phase 1 clinical tests of its cancer treatment candidate CUE-102.
The South Korean company said on Thursday the clinical trials will be conducted by its US partner Cue Biopharma Inc. on patients with stomach cancer, pancreatic cancer, ovarian cancer and colorectal cancer to confirm the drug’s safety, tolerability and efficacy.
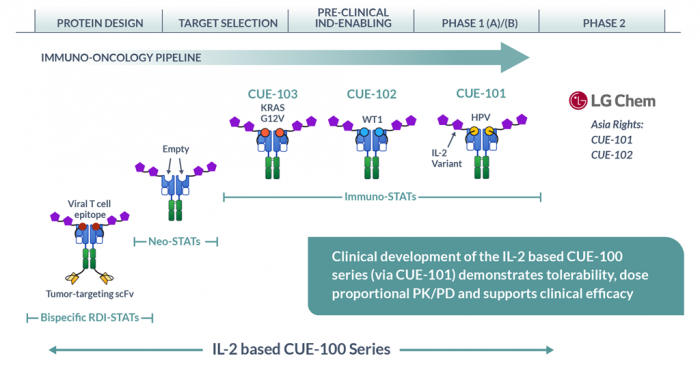
CUE-102 is one of three cancer immunotherapy candidates LG Chem and Cue Biopharma have jointly developed, based on the US biopharmaceutical company’s in-vivo immune function regulation platform, Immuno-STAT.
LG has the exclusive rights to develop and market the CUE-102 candidate in 11 Asian countries, including Korea, China and Japan, while the Boston-based biopharma has the rights in other countries.
LG Chem said if the phase 1 trials succeed, it will start phase 2 trials on cancer patients in Asian countries in the latter part of 2024.
OTHER DRUG CANDIDATES IN THE PIPELINE
Other LG drug candidates in the phase 1 trial stage include CUE-101, a candidate for head and neck cancer; a solid cancer treatment drug from Korean biotechnology firm Genome & Co.; and a non-small cell lung cancer treatment candidate from PDC*line Pharma SA, a French-Belgian biotech company.
LG Chem Life currently has a total of 12 drug candidates in the pipeline, of which four are anticancer therapies.
A gout treatment candidate, which LG is working on, has passed phase 2 tests in the US and is set to enter the final phase 3 trials with a plan to market in 2027. If the drug comes out as scheduled, it would be LG’s first new medicine in 15 years since its antidiabetic drug Zemiglo in 2012.
Write to Jae-young Han at jyhan@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaFutureChem seeks FDA nod for prostate cancer radiopharma trials
Bio & PharmaFutureChem seeks FDA nod for prostate cancer radiopharma trialsApr 20, 2022 (Gmt+09:00)
2 Min read -
 Bio & PharmaFDA to review Hanmi Pharmaceutical’s Rolontis for marketing approval
Bio & PharmaFDA to review Hanmi Pharmaceutical’s Rolontis for marketing approvalApr 13, 2022 (Gmt+09:00)
1 Min read -
 BiosimilarsSamsung Bioepis gets FDA approval for Lucentis biosimilar Byooviz
BiosimilarsSamsung Bioepis gets FDA approval for Lucentis biosimilar ByoovizSep 23, 2021 (Gmt+09:00)
3 Min read -
 ESG investmentLG Chem to invest $134 mn in incubator fund for firms with ESG tech
ESG investmentLG Chem to invest $134 mn in incubator fund for firms with ESG techMay 11, 2021 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


