Bio & Pharma
Medytox-Hugel dispute over botox strain intensifies with ITC probe
The investigation comes as Hugel awaits FDA approval for the US sale of Letybo
By May 03, 2022 (Gmt+09:00)
3
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



A legal dispute over a botox strain between South Korean pharmaceutical company Medytox Inc. and its crosstown rival Hugel Inc. has intensified with the International Trade Commission (ITC) officially launching an investigation into the case.
The US trade panel has begun looking into Medytox's complaint against Hugel, Croma-Pharma GmbH, its partner in the US and European markets, and Hugel America, their joint venture, according to Medytox on Tuesday.
In response, Hugel said it has hired a global law firm to handle the case, denying claims that it has poached Medytox’s botulinum toxin strain to make its medical products.
“The ITC’s investigation will lay bare Hugel’s illegal activities. Through this complaint, we want to protect our intellectual property rights and correct the malicious act of technology theft,” Medytox said in a statement.
Medytox said Quinn Emanuel Urquhart & Sullivan LLP will represent the company in the case against Hugel, Korea’s largest manufacturer of botulinum toxin, more commonly known as botox.
In late March, Medytox lodged a complaint with the ITC, asking the US trade panel to look into its claims and impose an import ban on Hugel’s botox product into the US.
Medytox said at the time that Hugel has produced its botox product, Letybo, by misappropriating trade secrets belonging to Medytox and is preparing to export Letybo to the US.

HUGEL SAYS CLAIMS ‘GROUNDLESS’
Hugel instantly rebutted Medytox’s claims, saying the accusations are “groundless.”
“The ITC initiating an investigation is the procedural routine. It doesn’t mean at all that Medytox’s claims have validity,” Hugel said in a statement. “We’ll squarely handle false claims filed against us.”
Hugel said it will take legal action in close cooperation with its largest shareholder, GS Holdings Co., and other key stakeholders such as Korean private equity firm IMM Investment and Singapore-based global healthcare investment fund CBC Group.
The complaint comes three years after Medytox filed a similar complaint with the ITC against Daewoong Pharmaceutical Co., another Korean botox maker.
In January 2019, Medytox lodged a complaint with the ITC against Daewoong, saying the competitor used Medytox’s botox strain to make its product, Nabota.
The ITC initially ordered a 21-month ban on imports of Daewoong’s botox product but later invalidated the ruling following a negotiated settlement of the matter between the two companies.
Under the settlement, California-based Evolus, the global distributor of Daewoong’s Nabota, offered $35 million in license fees, and a 16.7% Evolus stake to Medytox.
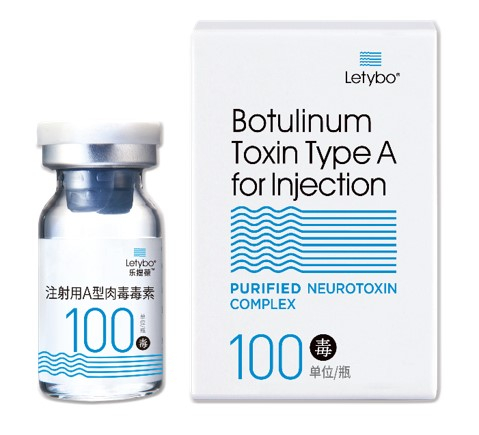
HUGEL AWAITS FDA MARKETING APPROVAL
Medytox suspects that all botox makers in Korea are using the botulinum strain that it secured from a US university laboratory in 1979.
However, Hugel and Daewoong have said they obtained their botulinum strains independently and so are different from Medytox's.
Industry watchers said the ITC’s investigation into Hugel may work against its plan to launch its botox product, Letybo, in the US market after obtaining regulatory approval by the end of this year.
“We have submitted all necessary documents on Letybo to the FDA for the final review of the product’s safety. This is separate from the ITC probe. We’re confident that we’ll get FDA marketing approval by year-end,” said a Hugel official.
Established in 2001, Hugel is Korea’s largest maker of botulinum toxin products used to treat overactive muscles and facial wrinkles. The company controls about half the Korean botox market.
Write to Sun-A Lee at suna@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaMedytox files complaint with ITC against Hugel over botox strain
Bio & PharmaMedytox files complaint with ITC against Hugel over botox strainApr 01, 2022 (Gmt+09:00)
3 Min read -
 Mergers & AcquisitionsGS ups spending to take control of botox maker Hugel
Mergers & AcquisitionsGS ups spending to take control of botox maker HugelFeb 13, 2022 (Gmt+09:00)
1 Min read -
 PharmaceuticalsMedytox’s US business outlook dim as contract with AbbVie ends
PharmaceuticalsMedytox’s US business outlook dim as contract with AbbVie endsSep 09, 2021 (Gmt+09:00)
1 Min read -
 PharmaceuticalsGS Group joins race to acquire Korea’s botox maker Hugel
PharmaceuticalsGS Group joins race to acquire Korea’s botox maker HugelJun 28, 2021 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


