Artificial intelligence
Vuno's brain imaging AI medical device secures FDA certification
The S.Korean startup aims to intensify the US dementia diagnosis market entry with its Vuno Med-DeepBrain
By Oct 10, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


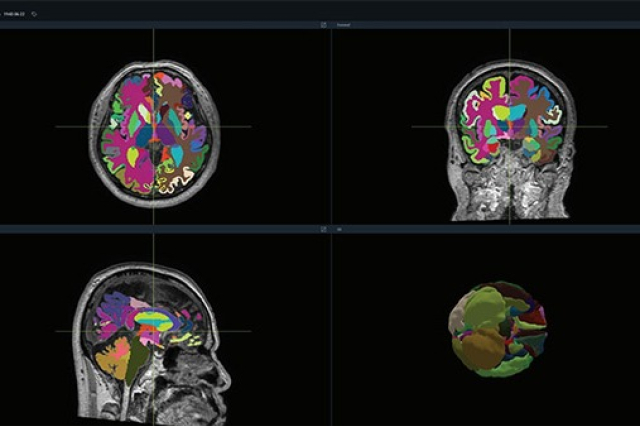
South Korea's medical device startup Vuno announced on Tuesday that its AI-powered brain quantification device Vuno Med-DeepBrain has received the 510(k) certification from the US Food and Drug Administration (FDA).
The 510(k) certification process, an FDA approval system, confirms the safety and effectiveness of medical devices by comparing them to previously certified products before they reach the market.
Leveraging deep learning, Vuno Med-DeepBrain analyzes brain MRI scans, segmenting the brain into over 100 distinct areas and rapidly quantifying atrophy in each region within a minute.
This state-of-the-art technology is set to assist healthcare professionals in diagnosing dementia that arises from major neurodegenerative conditions such as Alzheimer's and vascular dementia.
A significant implication of this technology is its potential to pinpoint patients at a higher risk of evolving from mild cognitive impairment into full-blown dementia.
With the FDA certification in hand, Vuno is gearing up to bolster its sales and marketing efforts targeting US medical institutions.
The timely FDA nod comes amid the projected surge in the early dementia diagnosis market, especially following this year's FDA approval of a new Alzheimer's treatment.
Vuno is strategically positioned to capitalize on this growth, planning to expand collaborations with enterprises eager for AI-driven brain MRI quantification solutions.
Earlier this year, Vuno highlighted Vuno Med-DeepBrain's capability in the early detection of Alzheimer's through its clinical research findings.
Data presented at the Alzheimer's Association International Conference (AAIC 2023) in July showcased the device's promise in diagnosing Alzheimer's in individuals exhibiting signs of subjective cognitive decline (SCD).
Write to Ye-Na Kim at yena@hankyung.com
More to Read
-
 Artificial intelligenceVuno's fundus-reading AI obtains medical device certification in Thailand
Artificial intelligenceVuno's fundus-reading AI obtains medical device certification in ThailandDec 15, 2022 (Gmt+09:00)
1 Min read -
 Artificial intelligenceVuno's AI-powered fundus reading solution approved for sale in Taiwan
Artificial intelligenceVuno's AI-powered fundus reading solution approved for sale in TaiwanNov 30, 2022 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


