FDA
Scope
Date
~
-
 Bio & Pharma
Bio & PharmaYuhan’s lung cancer drug clears FDA hurdle for global debut
A new lung cancer treatment developed by South Korea’s Yuhan Corp. has secured approval from the US Food and Drug Administration (FDA) as a co...
Aug 21, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion gets FDA nod for Keytruda copy's phase 3 clinical trials
Celltrion Inc., South Korea’s biosimilar giant, will proceed with phase 3 trials for global blockbuster drug Keytruda’s copycat CT-P51, ...
Aug 12, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaJLK gets FDA OK for prostate cancer diagnosis AI solution
South Korea’s JLK Inc., an AI-based diagnosis solution and platform maker, announced on Monday that it got approval from the US Food and Drug ...
Jun 25, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaInvestors dump Korean biotech HLB stocks on FDA rejection
HLB Co. and its affiliate stocks tumbled on Friday on news that the South Korean biotech group’s highly anticipated new cancer treatment Rivoc...
May 17, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaAprogen gets OK Phase 3 trials in India for Herceptin biosimilar
South Korea’s biopharmaceutical company Aprogen Inc. announced on Monday that its Phase 3 clinical trial for a biosimilar of Herceptin (trastu...
Feb 05, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaMedPacto gets FDA OK for colorectal cancer drug clinical trial
South Korea's biotech company MedPacto Inc. announced on Tuesday that it has got approval from the US Food and Drug Administration (FDA) for its I...
Jan 03, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCGBio gets FDA approval for Novosis Putty
South Korea's bio-regenerative medical firm CGBio announced on Tuesday that its Novosis Putty has been designated as a breakthrough device by the US...
Jan 02, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaToolGen gets FDA approval for TGT-001 as ODD
South Korean genome editing developer ToolGen Inc. announced on Monday that its application for orphan drug designation (ODD) of the gene correction...
Dec 18, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaGC Biopharma gets FDA approval for Alyglo
South Korea’s GC Biopharma Corp. announced on Monday that it received approval from the US Food and Drug Administration (FDA) for the immuno...
Dec 18, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaSK Bioscience applies Phase 3 trials in US for pneumococcal vaccine
South Korea's SK Bioscience Co. announced on Monday that it submitted the Investigational New Drug (IND) application for the Phase 3 clinical trial ...
Dec 11, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaSK Biopharm gears up to boost epilepsy drug sales in Europe
SK Biopharmaceuticals Co. is gearing up to explore the European market for its flagship epilepsy drug Cenobamate, while looking for new mergers and ...
Nov 16, 2023 (Gmt+09:00)
-
 Artificial intelligence
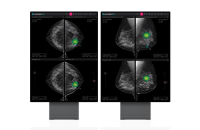
Artificial intelligenceLunit gets FDA approval for AI cancer diagnosis solution
South Korean medical AI company Lunit said on Tuesday its Lunit Insight DBT, an AI imaging diagnosis solution aiding in breast cancer diagnosis, has...
Nov 14, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaGencurix gets FDA OK for breast cancer prognosis analysis device
South Korean biotech company Gencurix Inc. announced on Wednesday its subsidiary GenoBio has received approval from the US Food and Drug Admini...
Nov 08, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaNKMax to perform first tests on dementia cell therapy in US
NKMax Co., South Korea's largest developer of natural killer (NK) cell therapies, will conduct its first clinical trials in the US for its candidate...
Oct 26, 2023 (Gmt+09:00)
-
 Bio & Pharma
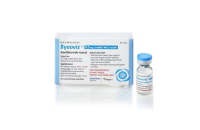
Bio & PharmaSamsung Bioepis gets FDA OK for its interchangeable biosimilar
Byooviz, a biosimilar of the macular degeneration treatment Lucentis and made by South Korea’s Samsung Bioepis, has received official design...
Oct 25, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion gets FDA approval for Remsima SC
South Korea's Celltrion Inc. announced on Monday it received a new drug approval for an autoimmune disease treatment Remsima SC (active ingredient i...
Oct 23, 2023 (Gmt+09:00)
-
 Artificial intelligence
Artificial intelligenceVuno's brain imaging AI medical device secures FDA certification
South Korea's medical device startup Vuno announced on Tuesday that its AI-powered brain quantification device Vuno Med-DeepBrain has received the 5...
Oct 10, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaYungjin Pharm's new drug candidate gets Fast Track from FDA
South Korea's Yungjin Pharm has secured Fast Track designation from the US Food and Drug Administration (FDA) for its new drug candidate KL1333, des...
Sep 06, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaHugel resubmits application to FDA for botulinum toxin product
South Korea's biopharmaceutical company Hugel Inc., announced on Friday that it has resubmitted applications to the US Food and Drug Administration ...
Sep 01, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaS.Korea's CGBio gets FDA approval for spinal implant
CGBio, a South Korean bio-regenerative medical company, announced on Monday that its "Advanced LumFix spinal fixation system," an implant and surgic...
Aug 14, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaRaphas microneedle passes US FDA's cGMP inspection
South Korea's microneedle maker Raphas Co. announced on Wednesday that it has passed the Current Good Manufacturing Practice (cGMP) inspection by ...
Jul 26, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaIldong Bioscience gets US FDA OK for probiotic raw materials
South Korea's Ildong Bioscience on Thursday said two of its probiotic raw materials received the certification generally recognized as safe (GRAS) f...
Jul 14, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaLegoChem Bio receives FDA approval for phase 1,2 trials of LCB84
South Korean pharmaceutical firm LegoChem Biosciences Inc. announced on Thursday that it has received approval from the US Food and Drug Administrat...
Jun 23, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaHuons’ 2% lidocaine gets approval from FDA
South Korean pharmaceutical firm, Huons Co., announced on Monday that it has secured another significant regulatory milestone in the US with the Foo...
Jun 19, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaHanmi Pharm's Efinopegdutide gets Fast Track Designation from FDA
Merck, known as MSD outside the United States and Canada, announced on Monday that Efinopegdutide, a technology transferred from the South Korean ph...
Jun 13, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion gets FDA approval for Humira biosimilar CT-P17
South Korean biopharmaceutical company Celltrion Inc. announced on Wednesday that it has obtained product approval from the US Food and Drug Adminis...
May 24, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaHLB submits new drug application to FDA for Rivoceranib liver cancer drug
HLB Co. announced on May 17 that it has submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) for the approval of Rivo...
May 19, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaFDA suspends COVID-19 diagnostic kit of S.Korea’s SD Biosensor
The US Food and Drug Administration (FDA) has recommended that consumers discard select models of Pilot COVID-19 self-diagnostic test kits sold on t...
May 08, 2023 (Gmt+09:00)
-
 Chemical Industry
Chemical IndustryCJ CheilJedang gets FDA approval for biodegradable packaging material
South Korea's largest food and bio company CJ CheilJedang Corp. announced on Thursday that its biodegradable material polyhydroxyalkanoate (PHA) has...
May 04, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaVerismo's CAR-T therapy designated fast-track status by FDA
Verismo Therapeutics, a US affiliate of South Korean pharmaceutical firm HLB Co., has received fast-track designation from the US Food and Drug Admi...
Apr 07, 2023 (Gmt+09:00)
